Microbiological Burden

As a recognized analytical and microbiological laboratory, Brightlabs offers extensive microbiological analyses to ensure the safety and quality of cannabis products. Microbiological contaminants, such as bacteria, yeasts, and molds, can pose serious health risks. The Total Aerobic Microbial Count (TAMC) and Total Yeast and Mold Count (TYMC) analyses provide a comprehensive overview of the number of bacteria, molds, and yeasts. A crucial factor in these analyses is the correct incubation time, ensuring accurate and reliable results.
TAMC and TYMC: What Are They?
TAMC (Total Aerobic Microbial Count): This measures the total number of aerobic bacteria in a sample. Aerobic bacteria are microorganisms that can grow in oxygen-rich environments. While some bacteria naturally occur on plants, high numbers can indicate contamination introduced during cultivation, processing, or storage. TYMC (Total Yeast and Mold Count): This measures the number of yeasts and molds in a product. Molds can produce mycotoxins, hazardous substances that pose health risks, particularly through inhalation. For instance Aspergillus, a type of mold that can lead to severe infections, such as Aspergillosis especially in patients with weakened immune systems.
Importance of Microbiological Analysis
Conducting microbiological tests on cannabis is crucial to comply with regulations and to prevent health risks for consumers. There are three main consequences of insufficient microbial control:
- Batch Rejection: If cannabis batches do not meet microbiological standards, they may be rejected. This results in significant financial losses for producers, as contaminated products must be destroyed. Research shows that microbial contamination is one of the leading causes of rejection in the cannabis industry (McPartland et al., 2017).
- Health Risks: Contaminated products can cause serious health problems, such as infections from molds like Aspergillus. Vulnerable groups, such as patients with chronic illnesses or weakened immune systems, are at particular risk (Verweij et al., 2020). Inhalation of mycotoxins can also cause toxic effects, including liver and kidney damage (Pitt & Hocking, 2009).
- Regulatory Issues: Producers who fail to meet microbiological limits risk penalties or loss of licenses. Compliance with strict microbiological standards is essential to ensure the integrity of production and sales (Thompson et al., 2021).
Incubation Times: A Critical Factor!
The incubation times for both TAMC and TYMC are supported worldwide by major regulatory agencies, including the United States Pharmacopeia (USP) and the European Pharmacopoeia (Ph. Eur.), which state that a minimum incubation of 5 days for TYMC and 3 days for TAMC is needed for reliable results (European Pharmacopoeia, 2024; United States Pharmacopeia, 2024). Figure 1: TYMC measurement at Brightlabs, day 1 to 5. Deviating from these parameters, such as incubation time, risks underreporting, as shown in figure 1.
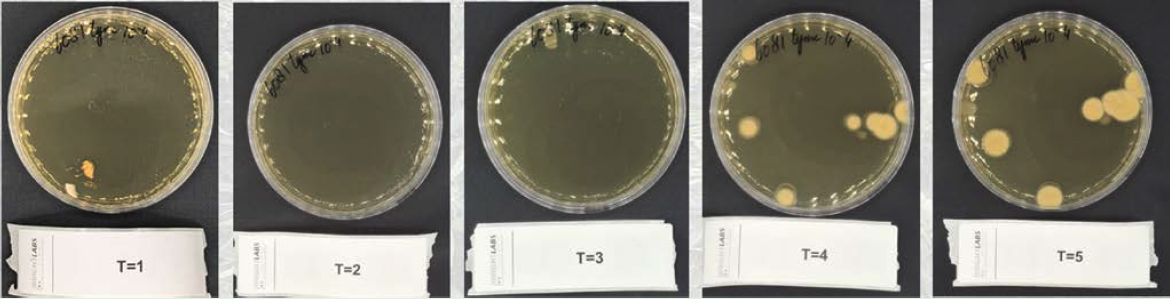
Figure 1: TYMC measurement at Brightlabs, day 1 to 5.
TAMC at Brightlabs
For TAMC, an incubation temperature of 30-35°C for 3-5 days is required to promote the full growth of aerobic bacteria. Shorter incubation times may disrupt the detection of slow-growing bacteria, leading to false-negative results (United States Pharmacopeia, 2023). It is also crucial to maintain the correct temperature, as temperatures that are too high can promote the growth of thermophilic bacteria, which are not representative of typical contaminants (World Health Organization, 2019). In figure 2, petri dishes are shown after day 5 of TAMC incubation conducted by Brightlabs, using dilution series ranging from 10-1 to 10-5. These dilutions are necessary to ensure an accurate count by preventing overgrowth (which would make the results uncountable)
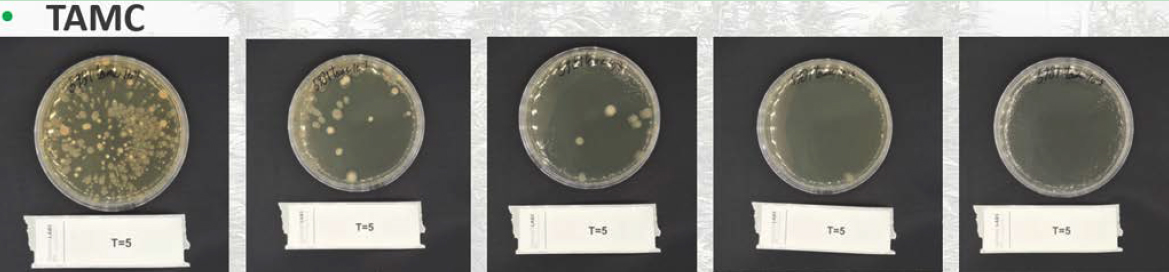
Figure 2: TAMC measurement at Brightlabs on day 5 ranging from 10-1 to 10-5
TYMC at Brightlabs
For TYMC, samples are incubated at 20-25°C for 5-7 days. These lower temperatures are crucial for cultivating slow-growing molds. Research by Rimek et al. (2019) shows that molds like Aspergillus require a longer incubation time to form visible colonies. Short incubation times can make yeasts and molds invisible, leading to underreporting of potentially dangerous organisms such as mycotoxinproducing molds (Keller et al., 2020). In figure 3, petri dishes are shown after day 5 of TYMC incubation conducted by Brightlabs, using dilution series ranging from 10-1 to 10-5.

Figure 3: TYMC measurement at Brightlabs on day 5 ranging from 10 -1 to 10 -5.
Conclusion
Brightlabs provides reliable microbiological analyses that meet the highest scientific and regulatory standards. Our accurate TAMC and TYMC analyses, supported by scientific research, minimize the risk of contamination and ensure that your cannabis products are safe and compliant. Contact us for more information about our extensive microbiological analysis capabilities.
References
- European Pharmacopoeia. (2024). Chapter 2.6.12 Microbial Examination of Non-Sterile Products: Total Aerobic Microbial Count (TAMC) andTotal Yeast and Mold Count (TYMC). Strasbourg: European Directorate for the Quality of Medicines & HealthCare.
- Keller, N. P., Turner, G., & Bennett, J. W. (2020). Fungal secondary metabolism-from biochemistry to genomics. Nature Reviews Microbiology, 3(12), 937-947. https://doi.org/10.1038/s41579-019-0317-5
- McPartland, J. M., Clarke, R. C., & Watson, D. P. (2017). Cannabis microbiota: A source of pathogens and toxins. Journal of Cannabis Research, 1(1), 1-12. https://doi.org/10.1186/ s42238-019-0004-3
- Pitt, J. I., & Hocking, A. D. (2009). Fungi and Food Spoilage. Springer Science & Business Media.
- Rimek, D., et al. (2019). Growth kinetics of Aspergillus species at different incubation times and temperatures. Journal of Clinical Microbiology, 37(9), 288-292. https://doi.org/10.1128/ JCM.37.9.288-292
- United States Pharmacopeia. (2024). Microbiological Examination of Nonsterile Products: Tests for Specified Microorganisms. United States Pharmacopeial Convention.
- Verweij, P. E., et al. (2020). Aspergillus infections associated with medicinal cannabis use. Emerging Infectious Diseases, 26(3), 355-357. https://doi.org/10.3201/eid2603.190470
- World Health Organization. (2019). Good Manufacturing Practices for Herbal Medicines.
Contact
Questions? Please feel free to contact us.

